MBI Videos
John Tyson
-
 John TysonOne of the basic characteristics of living organisms is their ability to process information about their external environment and internal state and to implement adaptive responses to the challenges they face. At the cellular level, these information processing tasks are carried out by complex networks of interacting genes and proteins; quite differently than the information processing done by digital computers or (analog) central nervous systems. Despite the triumphs of molecular biologists over the past 40 years in identifying and characterizing the components of these networks, their information-processing capabilities are still largely mysterious. Is there a basic theory of information-processing by molecular reaction networks that is biochemically realistic, reasonably accurate and comprehensive, and of predictive value? I will make the case that bifurcation theory of dynamical systems provides a framework for thinking about this problem. Briefly put, a one-parameter bifurcation diagram (dynamical variable as a function of control parameter) is the mathematical analog of the physiologist’s “signal-response� curve; and a two-parameter bifurcation diagram (e.g., physiological control parameter versus level of gene expression) can provide insight into the translation from genotype to phenotype. I will illustrate these principles with a number of classic examples from the field of network dynamics and cell physiology, and I will relate this particular problem to broader considerations of the “Rules of Life�.
John TysonOne of the basic characteristics of living organisms is their ability to process information about their external environment and internal state and to implement adaptive responses to the challenges they face. At the cellular level, these information processing tasks are carried out by complex networks of interacting genes and proteins; quite differently than the information processing done by digital computers or (analog) central nervous systems. Despite the triumphs of molecular biologists over the past 40 years in identifying and characterizing the components of these networks, their information-processing capabilities are still largely mysterious. Is there a basic theory of information-processing by molecular reaction networks that is biochemically realistic, reasonably accurate and comprehensive, and of predictive value? I will make the case that bifurcation theory of dynamical systems provides a framework for thinking about this problem. Briefly put, a one-parameter bifurcation diagram (dynamical variable as a function of control parameter) is the mathematical analog of the physiologist’s “signal-response� curve; and a two-parameter bifurcation diagram (e.g., physiological control parameter versus level of gene expression) can provide insight into the translation from genotype to phenotype. I will illustrate these principles with a number of classic examples from the field of network dynamics and cell physiology, and I will relate this particular problem to broader considerations of the “Rules of Life�. -
 John Tyson
John TysonThe physiological properties of living cells are determined by underlying networks of interacting genes, mRNAs, proteins and metabolites. These biochemical networks are staggeringly complex, highly nonlinear, dynamical systems that process information in time and space, in order to determine the optimal responses of a cell to challenging environmental conditions and its own internal damage-reporting mechanisms. To ferret out these interactions is a problem for molecular cell biologists, but to understand how biochemical networks coordinate cellular responses is a problem in applied mathematics. Using some simple examples of cellular decision-making (bistable switches) and time-keeping (limit cycle oscillations), I will show how dynamical systems theory and numerical simulations can shed considerable light on the molecular basis of cell physiology.
-
 John Tyson
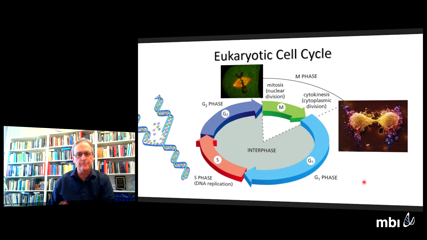
John TysonProgression through the eukaryotic cell cycle is controlled at a series of checkpoints guarding transitions from one phase of the cycle to the next, e.g., G1-to-S, G2-to-M, metaphase-to-anaphase. These checkpoints ensure that a cell has satisfied certain requirements that are necessary for success of the next phase, e.g., that any DNA damage is repaired before the cell replicates its chromosomes in S phase. These transitions are irreversible: as soon as the conditions of the checkpoint are satisfied, the cell proceeds to the next phase and does not subsequently back up to the immediately preceding phase. The irreversibility of these transitions gives the cell cycle its directionality (G1 → S → G2 → M → G1 ...). The genes and proteins governing these checkpoints have been discovered by molecular geneticists, but the mechanistic basis of irreversibility is still a subject of controversy. Many molecular biologists think that the transitions are irreversible because key proteins are chemically degraded at each transition, but we maintain that irreversibility is a consequence of bistability and hysteresis in the underlying regulatory network. To prove this claim, JJT will describe the mechanism of the G1-S transition in some detail, build and analyze a mathematical model of the mechanism, and compare the implications of the model to experimental facts.